Table of Contents
Learn about the mechanisms of the main corrosion types and which stainless steel alloys provide the best protection against them.
Corrosion resistance
Why does stainless steel have good corrosion resistance?
Chromium (Cr), in combination with oxygen (O), creates a thin film layer of Cr2O3 on the surface of the steel, which provides non-corrosive properties to the material. This layer blocks the oxygen’s diffusion to the steel surface and thus prevents corrosion from spreading into the bulk of the metal.
Chromium effect on corrosion resistance of stainless steel
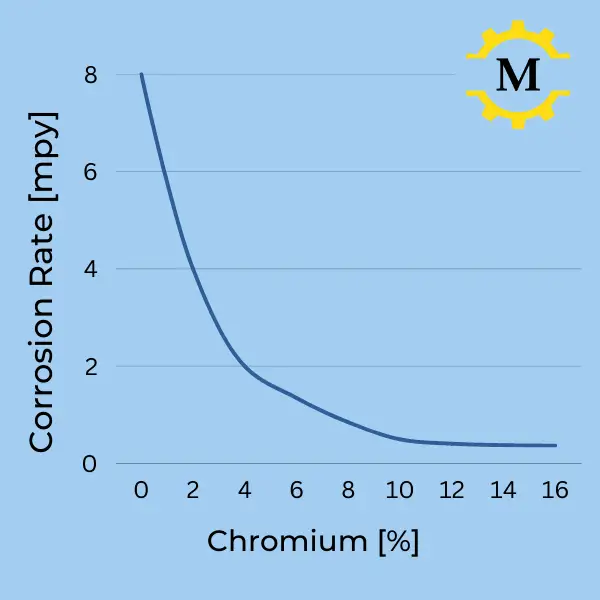
- A higher chromium content increases the alloy’s corrosion resistance.
- The chart on the left shows the corrosion rate in mpy (Millimeters per year) as a function of the chromium content.
- As you can see on the graph, a small amount of chromium already gives significant corrosion resistance, and above 10%, the effect flattens.
- Chromium alone provides protection in atmospheric and aqueous environments.
- Adding molybdenum increases the resistance to chloride penetration.
- Adding nickel improves the resistance in acid environments.
Which Stainless Steel alloy is effective in each corrosive environment?
| Stainless Steel | Atmospheric Environment | Aquatic Environment | Chemical Environment | |||||
| Mild | Industrial | Marine | Fresh Water | Salt Water | Mild | Oxidizing | Reducing | |
| 201 | V | V | V | V | V | V | ||
| 202 | V | V | V | V | V | V | ||
| 205 | V | V | V | V | V | V | ||
| 301 | V | V | V | V | V | V | ||
| 302 | V | V | V | V | V | V | ||
| 302B | V | V | V | V | V | V | ||
| 303 | V | V | V | V | ||||
| 303 Se | V | V | V | V | ||||
| 304 | V | V | V | V | V | V | ||
| 304L | V | V | V | V | V | V | ||
| 304 Cu | V | V | V | V | V | V | ||
| 304N | V | V | V | V | V | V | ||
| 305 | V | V | V | V | V | V | ||
| 308 | V | V | V | V | V | V | ||
| 309 | V | V | V | V | V | V | ||
| 309S | V | V | V | V | V | V | ||
| 310 | V | V | V | V | V | V | ||
| 310S | V | V | V | V | V | V | ||
| 314 | V | V | V | V | V | V | ||
| 316 | V | V | V | V | V | V | V | V |
| 316F | V | V | V | V | V | V | V | V |
| 316L | V | V | V | V | V | V | V | V |
| 316N | V | V | V | V | V | V | V | V |
| 317 | V | V | V | V | V | V | V | V |
| 317L | V | V | V | V | V | V | V | |
| 321 | V | V | V | V | V | V | ||
| 329 | V | V | V | V | V | V | V | V |
| 330 | V | V | V | V | V | V | V | V |
| 347 | V | V | V | V | V | V | ||
| 348 | V | V | V | V | V | V | ||
| 384 | V | V | V | V | V | V | ||
| 403 | V | V | V | |||||
| 405 | V | V | V | |||||
| 409 | V | V | V | |||||
| 410 | V | V | V | |||||
| 414 | V | V | V | |||||
| 416 | V | V | ||||||
| 420 | V | V | ||||||
| 420F | V | V | ||||||
| 422 | V | V | ||||||
| 429 | V | V | V | V | V | |||
| 430 | V | V | V | V | V | |||
| 430F | V | V | V | V | ||||
| 431 | V | V | V | V | V | |||
| 434 | V | V | V | V | V | V | ||
| 436 | V | V | V | V | V | V | ||
| 440A | V | V | V | |||||
| 440B | V | V | ||||||
| 440C | V | V | ||||||
| 442 | V | V | V | V | V | |||
| 446 | V | V | V | V | V | V | ||
types of Stainless Steel
| Property | Austenitic | Martensitic | Feritic | PH | Duplex |
|---|---|---|---|---|---|
| Corrosion Resistance | Excelent | Fair | Good | Good | Excelent++ |
| Magnetic? | No | Yes | Yes | No | No |
| Heat Treatable? | No | Yes | No | Yes | No |
| Machinability | 35-75% | 40-75% | 40-75% | 40-50% | 20-30% |
| Hardness | ~180 | Max 600 | ~200 | Max 400 | ~280 |
| Strength [Kpsi] | ~90 | ~120 | ~100 | ~200 | ~250 |
| Cr | 16-20% | 11-14% | 11-18% | 14-17% | 18-30% |
| Ni | 6-15% | 0-2% | 0-1% | 4-8% | 4-7% |
| Mo | 2-4% | - | 0-1.2% | 1.5-2.5% | 0-5% |
Corrosion Types
Learn about the mechanisms of the main corrosion types and which stainless steel alloys provide the best protect
Pitting

Pitting is a form of extremely localized corrosion that occurs When the protective chromium-oxide film breaks down in small isolated spots, such as when chlorides and fluorides have direct contact with the surface. Pitting can be avoided by using stainless steels containing molybdenum, such as Type 316 or 317. (Learn More)
Crevice Corrosion
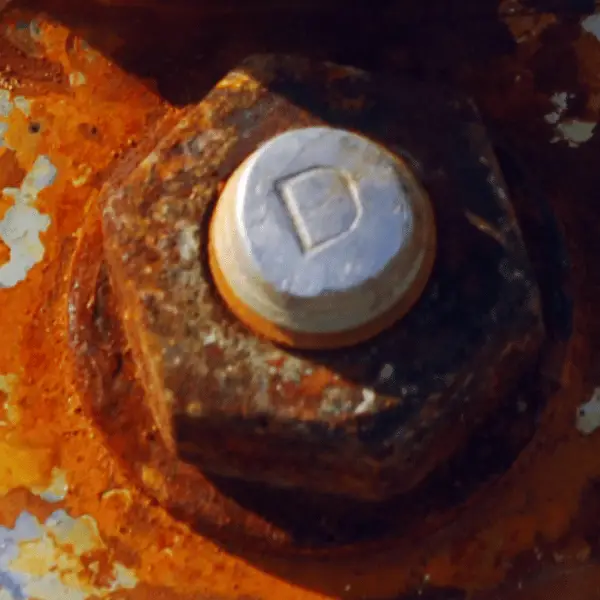
Crevice corrosion occurs from an attack on the surfaces by a stagnant solution in crevices. A typical case is a bolt or rivet heads, Where small amounts of liquid can accumulate. Once an attack begins, its progress is very rapid. Stainless steels containing molybdenum provide better resistance. (Learn More)
Stress Corrosion

Stress-Corrosion occurs by the combined effects of temperature and tensile stress in corrosive environments. Wet-dry or intermitted heat transfer conditions promote the concentration of chlorides and accelerate the development of stress-corrosion cracking. Duplex stainless steels have excellent stress corrosion resistance. Frenetic stainless steel types 405 and 430 are also recommended among the more common alloys. (Learn More)
Intergranular Corrosion
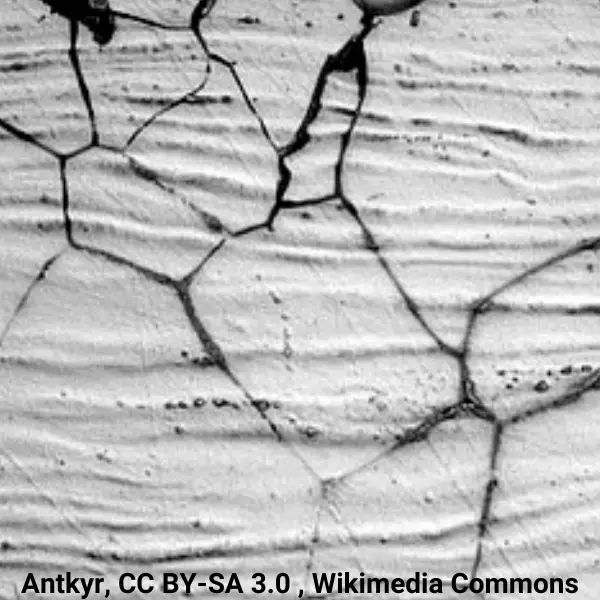
Occurs when austenitic stainless is heated or cooled through the temperature range of about 800-16500°F (450-900°C). The chromium along grain boundaries tends to combine with carbon creating carbide precipitation. Low-carbon alloys with an “L” suffix, such as 304L & 316L, are less sensitive because there is not enough carbon to react with the chromium. Alloys with the addition of columbium (SAE 347) or titanium (SAE 316Ti) are helpful due to their close affinity to carbon. (Learn more).
High Temperatur Corrosion (Scaling)

Scaling occurs when metal is heated to very high temperatures and exposed to oxygen. The metal corrodes and forms unwanted deposits (scaling). The maximum temperature to which the protective film still functions depends on the chromium content. Scaling is a common problem in oil and gas production, water transport systems, and Power generation equipment.
- Stainless alloys with less than 18% chromium are limited to temperatures below 1500°F (800C). This includes most frenetic stainless alloys such as 410, 416, or 420 and PH alloys such as 17-4PH.
- 18-20% chromium content protects up to 1800°F (980°C). This range contains the most popular austenitic stainless steels, such as 304 and 316.
- Alloys with more than 25% chromium, such as 309, 314, 452, and most duplex alloys, protect up to 2000°F (1,100°C)